ChatGPT is a versatile tool that can be leveraged to streamline processes and tasks within the field of quality management.
Did you know that professionals often invest in standard-specific checklists for quality audits? These audit checklists, for instance, for quality management systems in line with ISO 9001 or ISO 13485, range from 50 to 200 EUR in price. Generally, they are nothing more than a restatement of the standard's requirements in question format. Interestingly, this is a task that ChatGPT can perform efficiently.
Understanding Standards
Quality management standards comprise a collection of guidelines and principles intended to ensure that an organization's products, services, or processes consistently meet or exceed customer expectations. These standards provide a systematic approach to managing an organization's processes to produce high-quality outcomes.
ISO 9001, issued by the International Organization for Standardization (ISO), is a prime example of quality management standards. It outlines the criteria for a quality management system and focuses on customer satisfaction, top management's involvement, a process approach, and continual improvement.
Another example is ISO 13485, specifically designed for quality management systems in the medical devices sector. It ensures safety and effectiveness in the design, production, and delivery of medical devices.
The International Automotive Task Force (IATF) issues the IATF 16949 standard, which defines the requirements of a quality management system for automotive industry organizations.
In contrast, the AS9100 series is the standard for quality management systems in the aerospace industry, including manufacturing, maintenance, and distribution organizations.
Finally, the ISO/IEC 17025 standard is employed by testing and calibration laboratories. It addresses not only the quality management system but also includes technical competence requirements.
Standards like these assist organizations in consistently meeting customer and regulatory requirements while pursuing continual improvement in their quality management systems.
The Benefits of Using an Audit Checklist
An audit checklist is a useful tool for conducting a quality management system (QMS) audit based on standards such as ISO 9001 or similar. Here's why it's beneficial:
- Consistency and Thoroughness: An audit checklist provides a systematic and uniform approach for auditors to evaluate the QMS, ensuring all critical elements of the standard are reviewed and no vital aspects are overlooked.
- Efficiency: It serves as a roadmap for the audit process, potentially reducing the time and effort required for planning and conducting the audit.
- Facilitating Communication: It can act as a communication tool among audit team members, the auditee, and other stakeholders, ensuring transparency about what will be assessed and promoting understanding and cooperation.
- Documentation: The completed checklist serves as a record of the audit process, providing a clear snapshot of what was examined, the findings, and any necessary corrective actions.
- Training: For less experienced auditors, the checklist can be a learning tool, illustrating which aspects of the QMS need to be evaluated.
Remember, a checklist should not restrict auditors from exploring pertinent issues beyond those included in it. Instead, it should serve as a foundation for conducting a comprehensive and effective audit.
Utilizing ChatGPT to Craft an Audit Checklist
ChatGPT can be leveraged to create an audit checklist based on the standard itself. Let's illustrate this using the standard PS 9000:2016, which is freely available and therefore suitable for demonstration purposes.
PS 9000:2016 is a standard developed by the Pharmaceutical Quality Group (PQG) of the Chartered Quality Institute (CQI). It offers guidance for the manufacture of pharmaceutical packaging materials, including instructions on Good Manufacturing Practice (GMP).
The standard outlines the requirements for a Quality Management System (QMS) specifically for suppliers of packaging materials intended for medicinal product production and packaging. PS 9000:2016 is designed to work in conjunction with ISO 9001, enhancing the requirements of this standard with additional industry-specific guidance.
Though PS 9000:2016 is used here as an example, ISO 9001, ISO 13485, or any other standard can serve as a basis.
How to Formulate an Audit Checklist for PS 9000:2016
The aim is to feed ChatGPT with the text of the standards and use a suitable prompt to ask it to generate audit questions from the requirements of the standards.
ChatGPT can deliver outputs in various forms such as text, tables, JSON, CSV, and others. For this specific use case, a table or CSV format might be suitable.
Constructing the Prompt
The following prompt showcases how this idea can be implemented. In this case, we aim to develop audit questions for section 5.2 of PS 9000:2016.
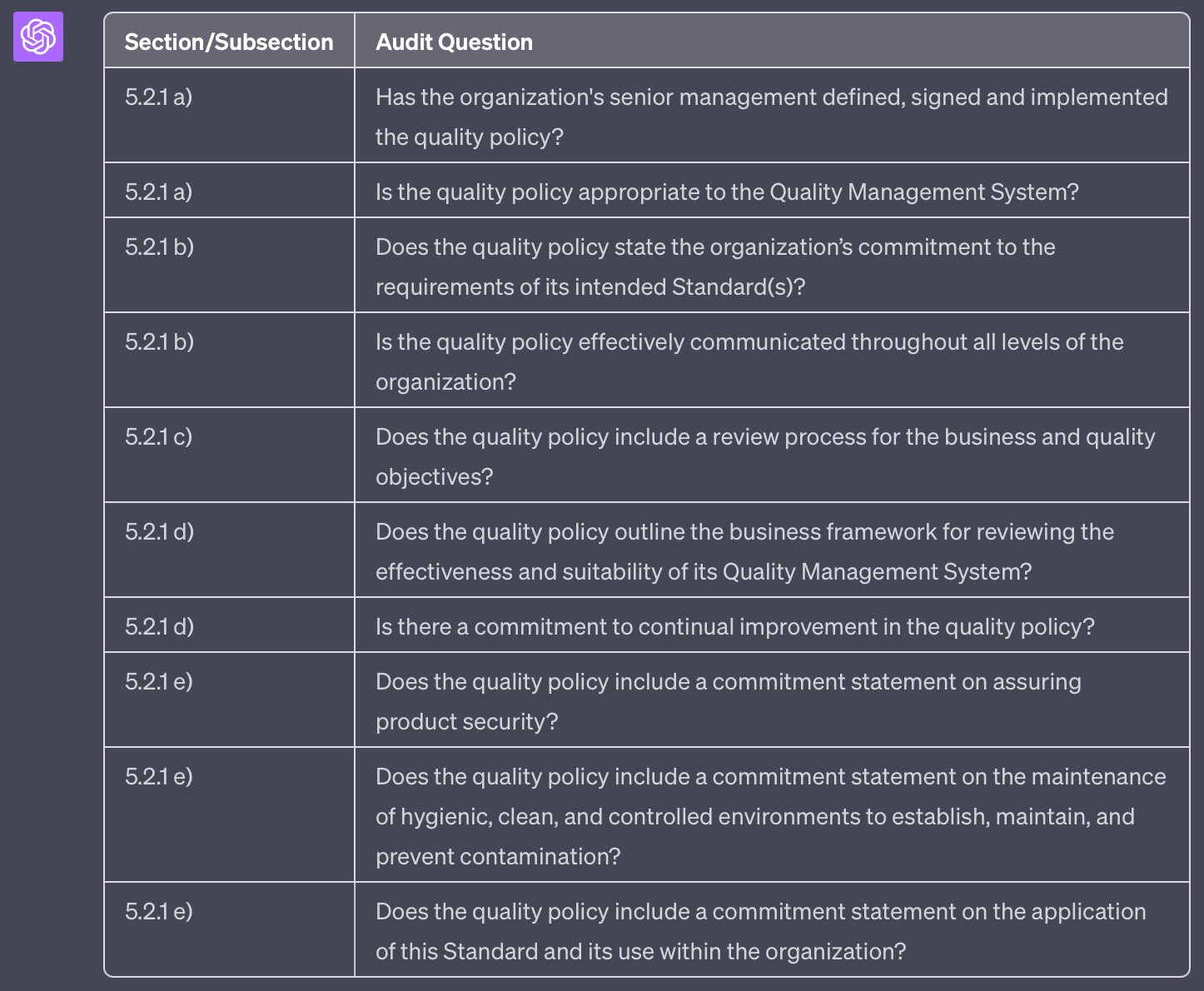
I ask you to generate questions for audit checklist. The checklist should support an audit against the standard PS 9000:2016. I will provide you with a text with requirements from this standards.
Please provide me with suitable audit questions covering this text. Provide your result in table format. The first column should refer to the section/subsection of the standard and the bullet point, if applicable. The second column should list the question. For each requirement there should be a new row in the table.
'''
5.2 Policy
5.2.1 Establishing the quality policy
a) The quality policy shall be defined, signed and implemented by the organisation’s senior management, ensuring the policy is appropriate to the Quality Management System.
b) The quality policy shall state the organisation’s commitment to the requirements of its intended Standard(s) and be effectively communicated throughout all levels of the organisation.
c) The quality policy shall include a review process for the business and quality objectives.
d) The quality policy shall outline the business framework for reviewing the effectiveness and suitability of its Quality Management System together with its commitment to continual improvement.
e) The quality policy shall include commitment statements on:
• assuring product security
• maintenance of hygienic, clean and controlled environments to establish and maintain and prevent contamination and
• application of this Standard and its use within the organisation
This prompt generates the following output:
The subsequent instructions detail the creation of a more comprehensive checklist that provides additional guidance for the audit.
I ask you to generate questions for audit checklist. The checklist should support an audit against the standard PS 9000:2016. I will provide you with a text with requirements from this standards.
Please provide me with suitable audit questions covering this text. Provide your result in table format.
The first column should refer to the section/subsection of the standard and the bullet point, if applicable.
The second column should list the question. For each requirement there should be a new row in the table.
The third column should list appropriate evidence documentation to be reviewed during the audit.
'''
5.2 Policy
5.2.1 Establishing the quality policy
a) The quality policy shall be defined, signed and implemented by the organisation’s senior management, ensuring the policy is appropriate to the Quality Management System.
b) The quality policy shall state the organisation’s commitment to the requirements of its intended Standard(s) and be effectively communicated throughout all levels of the organisation.
c) The quality policy shall include a review process for the business and quality objectives.
d) The quality policy shall outline the business framework for reviewing the effectiveness and suitability of its Quality Management System together with its commitment to continual improvement.
e) The quality policy shall include commitment statements on:
• assuring product security
• maintenance of hygienic, clean and controlled environments to establish and maintain and prevent contamination and
• application of this Standard and its use within the organisation
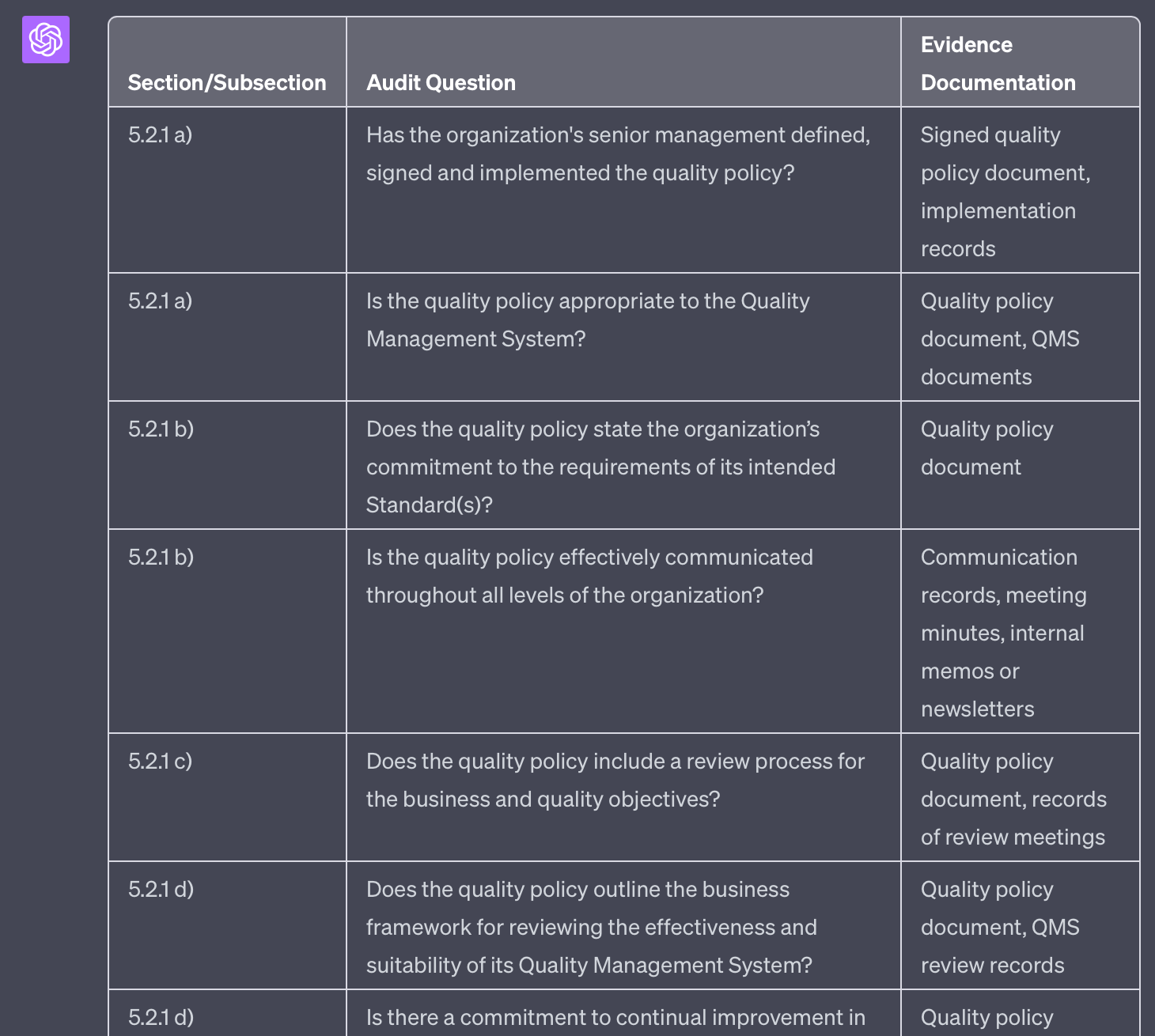
Please note that ChatGPT's standard output is formatted in Markdown. We can specify an alternative output format like this:
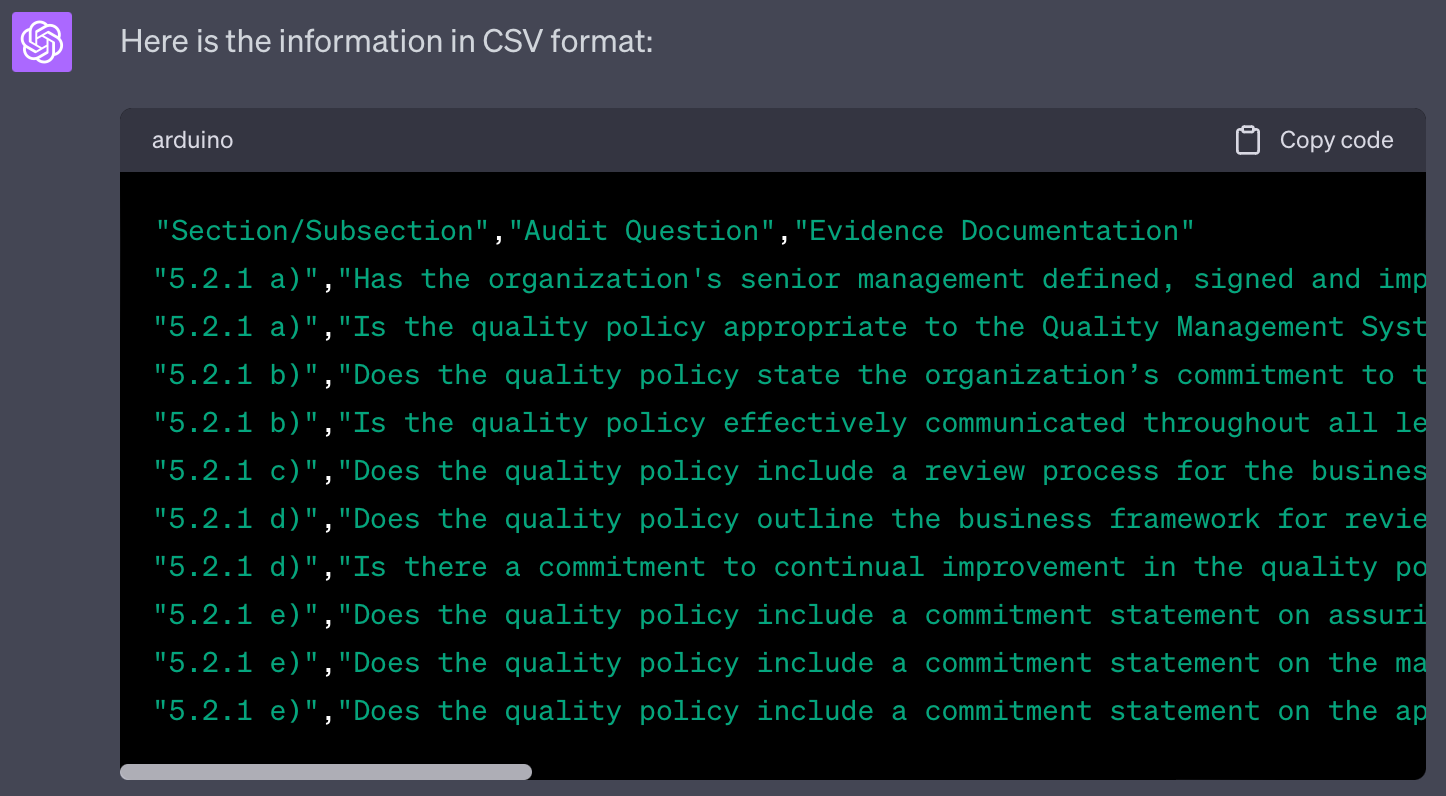
I ask you to generate questions for audit checklist. The checklist should support an audit against the standard PS 9000:2016. I will provide you with a text with requirements from this standards.
Please provide me with suitable audit questions covering this text. Provide your result in table format.
The first column should refer to the section/subsection of the standard and the bullet point, if applicable.
The second column should list the question. For each requirement there should be a new row in the table.
The third column should list appropriate evidence documentation to be reviewed during the audit.
Please provide the result as csv
This approach will yield results in Comma-separated value (CSV) format, which can be copied to a text file and opened in Excel or utilized in other applications.
Conclusion
ChatGPT is capable of transforming requirements defined in standards into Audit Checklist questions, producing outputs suitable for diverse applications.


