Navigating the Importance and Determination of Packaging Shelf-Life in the Pharmaceutical Industry
Reflecting on the presentation I delivered at the ECA conference on "Pharmaceutical Packaging Systems - Quality Control" on 30 November 2022, the importance of understanding the shelf-life of pharmaceutical packaging components is a crucial takeaway I emphasized.
The GMP Rationale: Why Define Shelf-Life?
The shelf-life of packaging materials is not just a detail—it's a vital part of Good Manufacturing Practice (GMP) compliance. The EU GMP Chapter 4 underlines this, requiring defined storage periods for starting and primary or printed packaging materials.
Beyond the regulatory necessity, let's dig deeper into the practical reasons for a defined shelf-life. Packaging materials age over time. This aging can alter their properties and potentially compromise their ability to protect the enclosed drug. Not just the physical materials, but also the packaging and labeling elements can be affected.
These materials must meet their functional and safety requirements throughout storage, processing, and until the end of the shelf-life of the drug they enclose. Hence, it's crucial to define and consider shelf-life for all pharmaceutical packaging components.
Decoding Shelf-Life: What Does it Mean?
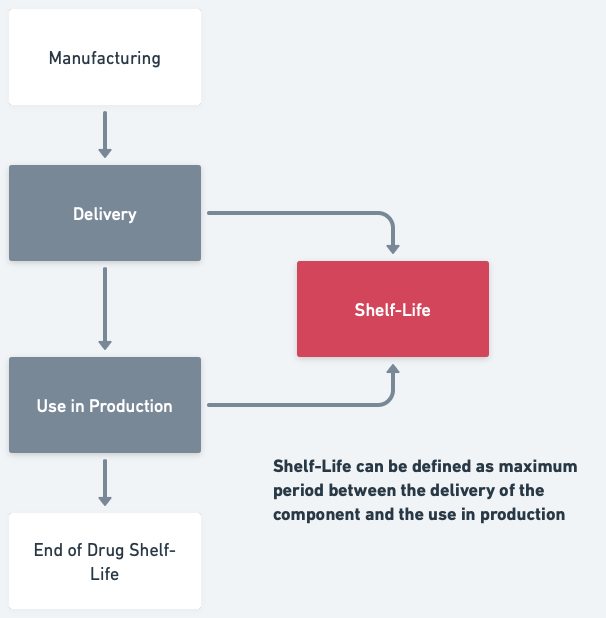
Shelf-life, in the context of pharmaceutical packaging, can be defined as the maximum period between the delivery of the component and its use in production. But remember, this definition isn't set in stone. Different organizations may use different definitions based on their unique needs and regulatory environments.
The advantage of utilizing the time between delivery and utilization in production is that it becomes relatively straightforward to ascertain the delivery date within the systems.
Detecting the Shelf-Life: Uncovering the Sources
How do we identify the shelf-life of pharmaceutical packaging components? A few key sources can help us.
Own experiences, including past complaints, deviations, and re-examination results, provide a valuable foundation. Supplier recommendations can add another layer of insight, offering industry-standard shelf-life data.
Literature reviews and learnings from other companies can also be useful. The shared experience within the pharmaceutical industry can contribute to a broader understanding of packaging material aging.
The Process: How to Define the Shelf-Life of Packaging Components
Defining the shelf-life for pharmaceutical packaging components is a systematic process that involves multiple steps.
- Define a Categorization of Packaging Components: Begin by classifying similar packaging components into distinct categories. This enables more streamlined and consistent shelf-life definition.
- Define Total Shelf Life: This refers to the absolute shelf-life of the packaging material, even after re-examination, if applicable. It's the longest period during which the component can maintain its quality and functionality.
- Decide on Re-examination: Determine if re-testing within the shelf-life is needed. This is often the case if the total shelf-life exceeds 2-3 years, as properties may change over an extended period.
- Set the Time Point for Re-examination: If re-examination is necessary, establish the timing for this process within the shelf-life period.
It's important to note that EU GMP Chapter 4 mandates the definition of "The maximum period of storage before re-examination," underscoring the importance of these steps.
When considering re-examination or re-testing, remember that not all properties of packaging components are subject to aging. Thus, the specifications for re-testing might mirror those for incoming goods testing, but with a focus on aspects likely to change during storage.
Typically, re-testing includes an identity test, which is a visual examination to ensure the correct material has arrived at the QC lab. Following that, a reduced scope of testing is performed, concentrating on what could have altered during storage.
Don't forget the transportation and storage packaging of the material. These external factors can also affect the condition of the packaging components and should be part of the shelf-life consideration.
Establishing a defined shelf-life isn't just about meeting regulatory requirements—it's a vital step in ensuring the safety, efficacy, and reliability of the drugs we deliver. In the pharmaceutical industry, understanding and implementing these steps is a shared responsibility, one that directly impacts patient outcomes.
Now, I'd love to hear your insights. How have you approached defining the shelf-life of packaging components in your work? What challenges and solutions have you encountered?


